bet isotherm|BET (Brunauer : Tuguegarao The BET isotherm, which extends both the Langmuir and Freundlich concepts, is especially valuable for systems where multilayer adsorption occurs. The BET equation, as previously discussed, is regarded as a more complex and comprehensive . Join the Reforged Discord - https://discord.gg/reforged Youtube Member - https://www.youtube.com/channel/UC45a_j76kNDwJvbc2ufOkJg/join .
bet isotherm,
Brunauer–Emmett–Teller (BET) theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of materials.
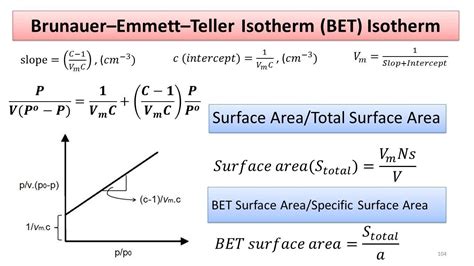
bet isotherm BET (Brunauer A type II isotherm (Figure \(\PageIndex{7}\) ) is very different than the Langmuir model. The flatter region in the middle represents the formation of a monolayer. A type II isotherm is obtained when c > 1 in the BET equation. This is the most common isotherm obtained when using the BET technique.BET Theory extends the Langmuir theory from monolayer adsorption to multilayer adsorption. © 2018 HORIBA, Ltd. All rights reserved.6. We use the BET equation to determine the monolayer absorbed gas volume (v. m) 1 𝑣𝑣[(𝑝𝑝0/𝑝𝑝) – 1] = 𝑐𝑐−1 𝑣𝑣𝑚𝑚𝑐𝑐 𝑝𝑝 𝑝𝑝0. + 1 𝑣𝑣𝑚𝑚𝑐𝑐 𝑣𝑣= adsorbed gas quantity. 𝑝𝑝0= saturation pressure of adsorbate.

The BET isotherm, which extends both the Langmuir and Freundlich concepts, is especially valuable for systems where multilayer adsorption occurs. The BET equation, as previously discussed, is regarded as a more complex and comprehensive .BET adsorption Isotherm BET Theory put forward by Brunauer, Emmett and Teller explained that multilayer formation is the true picture of physical Adsorption. One of the basic assumptions of Langmuir Adsorption Isotherm was that adsorption is monolayer in nature.

BET adsorption isotherm proposed by Brunauer, Emmett, and Teller accounts for multilayer adsorption. According to the theory, adsorption takes place only on specific areas of the sample surface (one per molecule), and doesn’t stop at monolayer formation but first adsorbed gas molecules provide an adsorption site for subsequent gas molecules .
BET (BrunauerFor molecules in contact with a solid surface at a fixed temperature, the Langmuir Isotherm, developed by Irving Langmuir in 1916, describes the partitioning between gas phase and adsorbed species as a function of applied pressure.
bet isothermIf BET equation, when P/P0<<1 and c>>1, then it leads to monolayer formation and Type I Adsorption Isotherm is obtained. The reversible Type II isotherm is the normal form of isotherm obtained with a non-porous or macroporous adsorbent. The Type II isotherm represents monolayer-multilayer adsorption.
bet isotherm|BET (Brunauer
PH0 · Derivation of the Langmuir and BET Isotherms
PH1 · BET theory
PH2 · BET Theory and how its used to calculate surface area
PH3 · BET Theory
PH4 · BET Adsorption Isotherm Equation and Applications
PH5 · BET (Brunauer–Emmett–Teller)
PH6 · BET (Brunauer
PH7 · Adsorption and Desorption Isotherms
PH8 · Adsorption Isotherm and its Types
PH9 · 2.3: BET Surface Area Analysis of Nanoparticles